Reaching Every Corner of the Globe
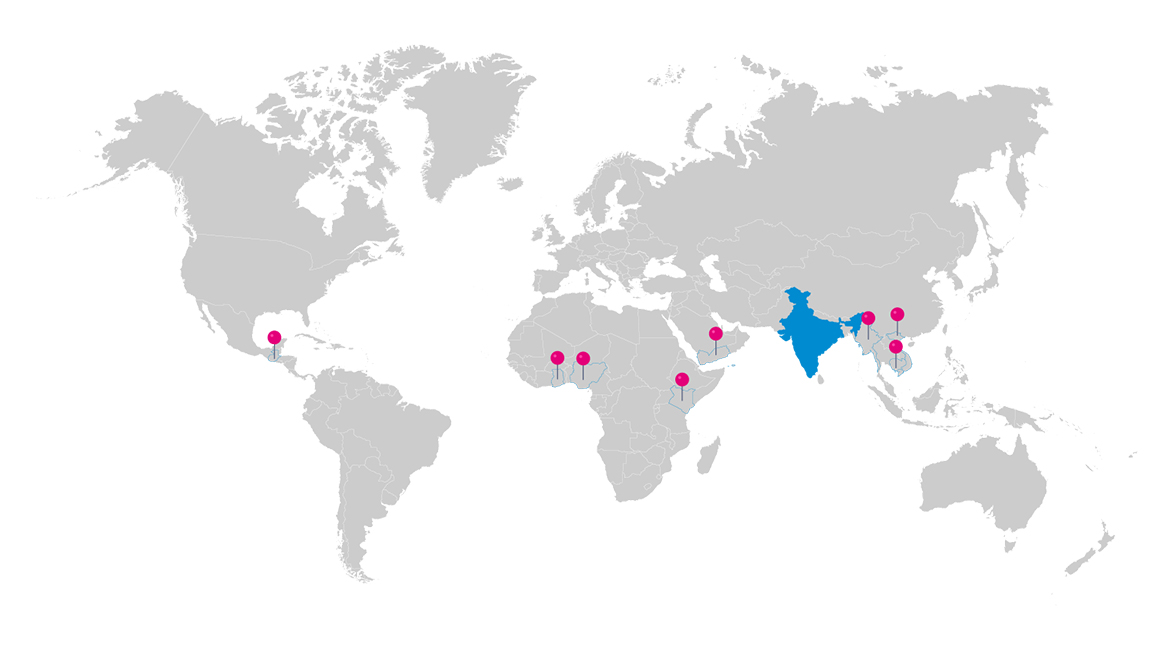
Strategic partnerships are integral to Sarian Healthcare's business model and crucial for delivering premium products to diverse local and international markets. These alliances uphold our high standards and bolster our global export capabilities, underscoring our unwavering commitment to excellence.
REGULATORY SUPPORT –
From preparing and submitting comprehensive dossiers to fulfilling technical requirements and staying updated with evolving global regulations, across Asia, Africa, and Central America, Our team ensures that every document aligns with the specific demands of health authorities across various countries. We meticulously handle the end-to-end process for registrations, approvals, and compliance, streamlining the path for smooth product launches in diverse markets.
By ensuring that our dossiers meet stringent international regulatory standards, we accelerate approval times and foster trust and long-term partnerships with global health agencies. Our focus on regulatory excellence enables us to expand into new territories while ensuring the highest level of quality for our products.
As one of the leading pharmaceutical companies accredited with ISO, WHO-GMP certifications, we ensure the highest standards of quality and compliance. With a strong global presence in key markets across Asia, Africa, and Central America, we are poised for expansion into even more diverse regions.


